Abstract
Background: Cold agglutinin disease (CAD) is a rare autoimmune disease, accounting for 15-25% of autoimmune hemolytic anemias (AIHA). The involved IgM autoantibodies, known as cold agglutinins (CA), bind to their antigen on red blood cells (RBCs) at an optimum temperature of 3-4°C inducing complement activation and formation of complement split products C3a and C3b. On rewarming to 37°C in the central circulation and detachment of CA, C3b remains bound and C3b-opsonized RBCs undergo phagocytosis by the mononuclear phagocytic system, mainly in the liver (extravascular hemolysis). To a lesser extent, complement activation proceeds until the formation of the membrane attack complex causing intravascular hemolysis (Berentsen S. Front Immunol 2020).
Pegcetacoplan is a pegylated pentadecapeptide that targets complement C3 and has the potential to prevent C3-mediated intravascular and extravascular hemolysis. In a phase 2, open-label, prospective pilot study conducted in subjects with a diagnosis of warm AIHA (n=12) or CAD (n=13) (NCT03226678), pegcetacoplan produced consistent, meaningful, and prolonged effects on most relevant clinical efficacy measures along with an acceptable safety profile in patients with CAD, supporting further clinical development of pegcetacoplan in this indication (Gertz M et al. EHA 2019: S899).
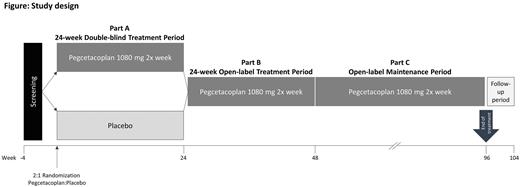
Study design and methods: The CASCADE study is a randomized, double-blind, placebo-controlled multicenter phase 3 study (NCT05096403) evaluating the efficacy and safety of pegcetacoplan in patients with primary CAD.
Patients are randomized in a 2:1 ratio to either receive pegcetacoplan 1080 mg subcutaneous injection using an infusion pump twice weekly for 24 weeks or placebo (figure). All patients who complete the 24-week double-blind period (part A) are eligible for an open-label treatment period (part B) with pegcetacoplan up to Week 48 and may subsequently continue to receive pegcetacoplan for an additional maintenance period (part C). After this, or if patients discontinue pegcetacoplan early, an end of treatment visit is performed, followed by an end of study visit 8 weeks later.
The main inclusion criteria are age ≥18 years; diagnosis of primary CAD; hemoglobin (Hb) level ≤ 9 g/dL, documented bone marrow biopsy result within 1 year of screening; and to either have or agree to receive vaccination against Streptococcus pneumoniae, Neisseria meningitidis (Types A, C, W, Y, and B), and Haemophilus influenzae (Type B).
The main exclusion criteria include having received other anti-complement therapies (approved or investigational) within 5 half-lives prior to randomization; rituximab monotherapy within 12 weeks prior to randomization, or rituximab combination therapies within 16 weeks prior to randomization; history of an aggressive lymphoma or presence of a lymphoma requiring therapy; having received an organ transplant; and cold agglutinin syndrome secondary to Mycoplasma pneumoniae, Epstein-Barr virus or other infection.
The primary endpoint is response defined as Hb level increase of ≥1.5 gram per deciliter (g/dL) from baseline, which is maintained from Week 16 through Week 24 in absence of blood transfusion from Week 5 through Week 24.
Secondary endpoints include change from baseline (CFB) to Week 24 in Hb level; transfusion avoidance from Week 5 to Week 24; CFB to Week 24 in Functional Assessment of Cancer Therapy-Anemia/Fatigue (FACT-An) Scale Score; CFB to Week 24 in markers of hemolysis (lactate dehydrogenase, haptoglobin level, indirect bilirubin level, absolute reticulocyte counts); CFB to Week 24 in 12-item short form survey (SF-12); CFB to Week 24 in five level EuroQol five dimensions questionnaire (EQ-5D-5L); and number of packed red blood cell transfusions from Week 5 to Week 24.
Statistics: The primary endpoint will be analyzed using a 2-sided Fisher's exact test at a significance level of 5%.
Study Status: The study is ongoing and plans to enroll 57 patients in the US, Europe, and Japan.
Disclosures
Jilma:Sanofi: Consultancy, Honoraria, Other: travel costs and lectures. Roman:Apellis: Speakers Bureau; Novartis: Speakers Bureau. Ueda:Alexion Pharma: Consultancy, Honoraria, Speakers Bureau; Novartis Pharma: Consultancy, Honoraria, Speakers Bureau; Chugai Pharma: Consultancy, Research Funding; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mappa:Sobi: Current Employment. Szamosi:Sobi: Current Employment, Current equity holder in publicly-traded company. Savage:Apellis Pharmaceuticals: Current Employment. Roeth:Sanofi: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Apellis Pharmaceuticals: Consultancy, Honoraria; Biocryst: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding.
OffLabel Disclosure:
Pegcetacoplan is a targeted C3 therapy designed to regulate excessive activation of the complement cascade. Pegcetacoplan is approved in the EU for the treatment of adults with paroxysmal nocturnal haemoglobinuria (PNH) who are anaemic after treatment with a C5 inhibitor for at least three months and in the United States for the treatment of adults with PNH. The therapy is also under investigation for several other rare diseases across haematology, nephrology, and neurology.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal